Internal energy of a body is the sum of all kinetic and all potential energies of its component particles.
Every body or any matter has tiny particles. Depending on the type of material and external influences, those constituent particles have a certain arrangement and distribution density. It applies to all of them:
- Particles are constantly in motion, which is why they possess kinetic energy.
- Particles constantly interact with each other, which is why they possess gravitational potential energy.
The sum of all the energies of all the particles that make up the body represents the energy of the body.
Connection of internal energy with physical quantities
The energy of a body is directly related to its temperature. When the temperature increases, the particles increase their speed of movement. Bearing in mind that with an increase in speed, the kinetic energy of a body also increases, it follows that an increase in energy will also occur.
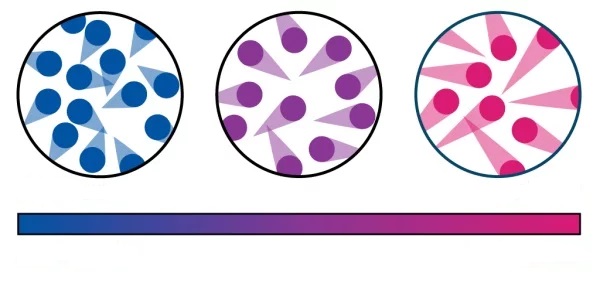
It follows that it depends directly on the temperature. The higher the temperature, the higher the internal energy, and conversely, the lower the temperature, the lower the internal energy of the body.
The temperature, for its part, is related to the physical quantities of pressure and volume, which means that the energy also indirectly depends on the changes of the body that refer to its volume and the pressure that can act on the body itself.
Often, the internal energy of a particular substance can be harnessed. It is an energy that people can manipulate to their advantage. You should know all the important information about this type of energy. It is really important if you want to understand all other energy types!
Follow the information and materials that will be published in the future. Complete connection with the www.matematikazasite.com/en profiles on Facebook, and Youtube using the buttons below.

 Please wait...
Please wait...

